Clinical Trials
This page provides general information about the phases of clinical trials and shouldn’t be taken in any form as medical advice.
If you’re looking for information about clinical trials at UCLA, please visit UCLA Health’s clinical trial search tool or call the hotline at 855-731-6040.
Our For Patients & Families page includes information about clinical trials beyond UCLA and guidance on what to consider when looking for stem cell clinical trials.
Overview
All research that takes place at the UCLA Broad Stem Cell Research Center is rooted in the ultimate goal of bringing new therapies to patients. Potential therapies are tested carefully in a stepwise fashion to evaluate safety and effectiveness, first in the lab, then using preclinical models and finally through a series of clinical trials.
At the BSCRC, our infrastructure, cutting-edge technologies and culture of collaboration help center investigators advance their discoveries through the multi-step clinical translation process as rapidly as possible. Over the past 20 years, our scientists have taken a number of stem cell-based therapies to clinical trials that have shown promising results in treating patients with cancer, blinding eye diseases, blood and immune diseases, and they are committed to continue translating promise to progress.
What's a clinical trial?
A clinical trial is a research study conducted with human participants to evaluate the safety and effectiveness of new medical treatments, interventions, drugs or medical devices.
Clinical trials occur in four phases
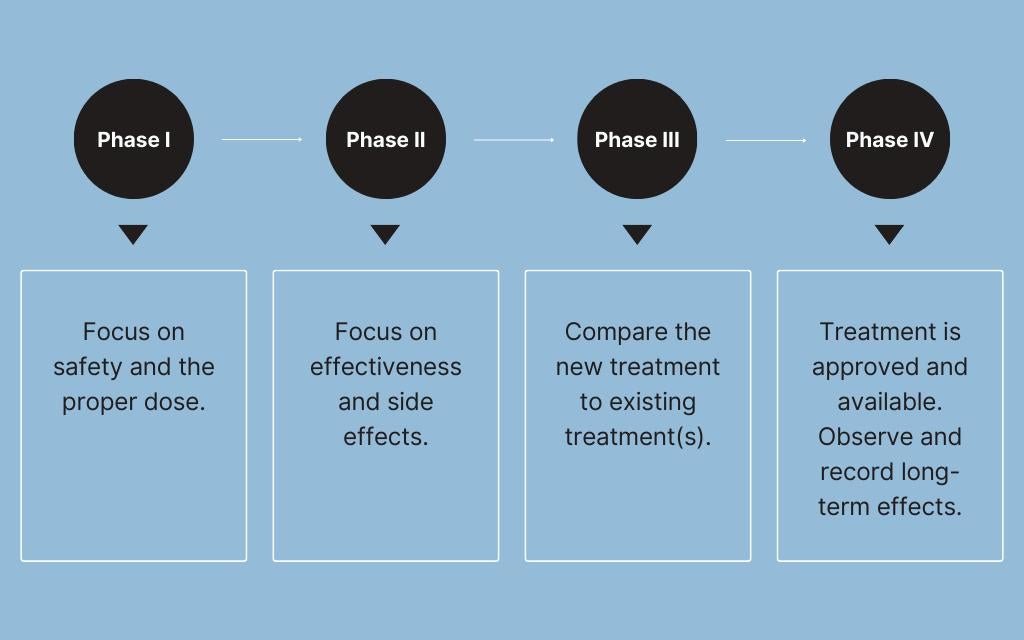
Phase I: Is it safe?
The objective of Phase I clinical trials is to evaluate the safety, dosage range and potential side effects of a therapy in a small group of humans. These trials are traditionally conducted in healthy volunteers. However, the unique characteristics and potential risks associated with cell and gene therapies make it more appropriate to conduct these early trials in individuals who have the disease or condition the therapy is ultimately supposed to treat. This approach allows researchers to gather relevant safety and efficacy data in a more representative context before moving on to later-phase trials. If a study isn’t deemed safe, it won’t move on to the next phase. This includes monitoring for adverse events, which are any unexpected health problems that might occur.
Phase II: Is it safe to continue testing and does it work?
After researchers have assessed safety in a limited number of subjects, the next phase of trials assesses a treatment's efficacy and side effects in a larger group of patients. Phase II trials typically involve a few hundred participants and are designed to help determine the optimal dosage and identify common short-term side effects.
Phase III: Is it safe and effective in a broader population?
To confirm a treatment's effectiveness, monitor side effects and compare it to standard treatments or a placebo, researchers then conduct trials with much larger numbers of participants. Phase III trials may involve several hundred to several thousand participants. This phase provides essential data for regulatory approval and is pivotal in shaping the drug's labeling and usage instructions.
Phase IV: What are the long-term benefits and risks?
In order to monitor the long-term safety and effectiveness of a treatment, trials continue even after a treatment has been approved and marketed to the public. Phase IV involves continued monitoring of a large patient population to detect rare or long-term side effects that may not have been apparent during earlier phases.
Note
The structure of the clinical trial process varies from country to country and depends on the type of intervention that's being evaluated (drugs, devices, vaccines, etc.). As research technologies and methods advance, these traditional phases in clinical research could also change. For example, the U.S. Food and Drug Administration’s Accelerated Approval Program allows for therapies that treat serious conditions and fill an unmet medical need, including many of those being developed at our center, to get approved faster if they meet certain criteria. However, under this program, treatments are conditionally approved and still need more testing to show they actually provide a clinical benefit.
Throughout the entire process, ethical considerations and regulatory oversight are critical to ensure the well-being of clinical trial patients and the integrity of the research. The number of patients in a trial is carefully determined to balance the need for scientific rigor with ethical considerations.
If you’re looking for information about clinical trials at UCLA, please visit UCLA Health’s clinical trial search tool or call the hotline at 855-731-6040.