Overview
The Microscopy Core is a collaboration between our center and the Department of Molecular, Cell and Developmental Biology. The core's mission is to promote interdisciplinary and collaborative research across UCLA.
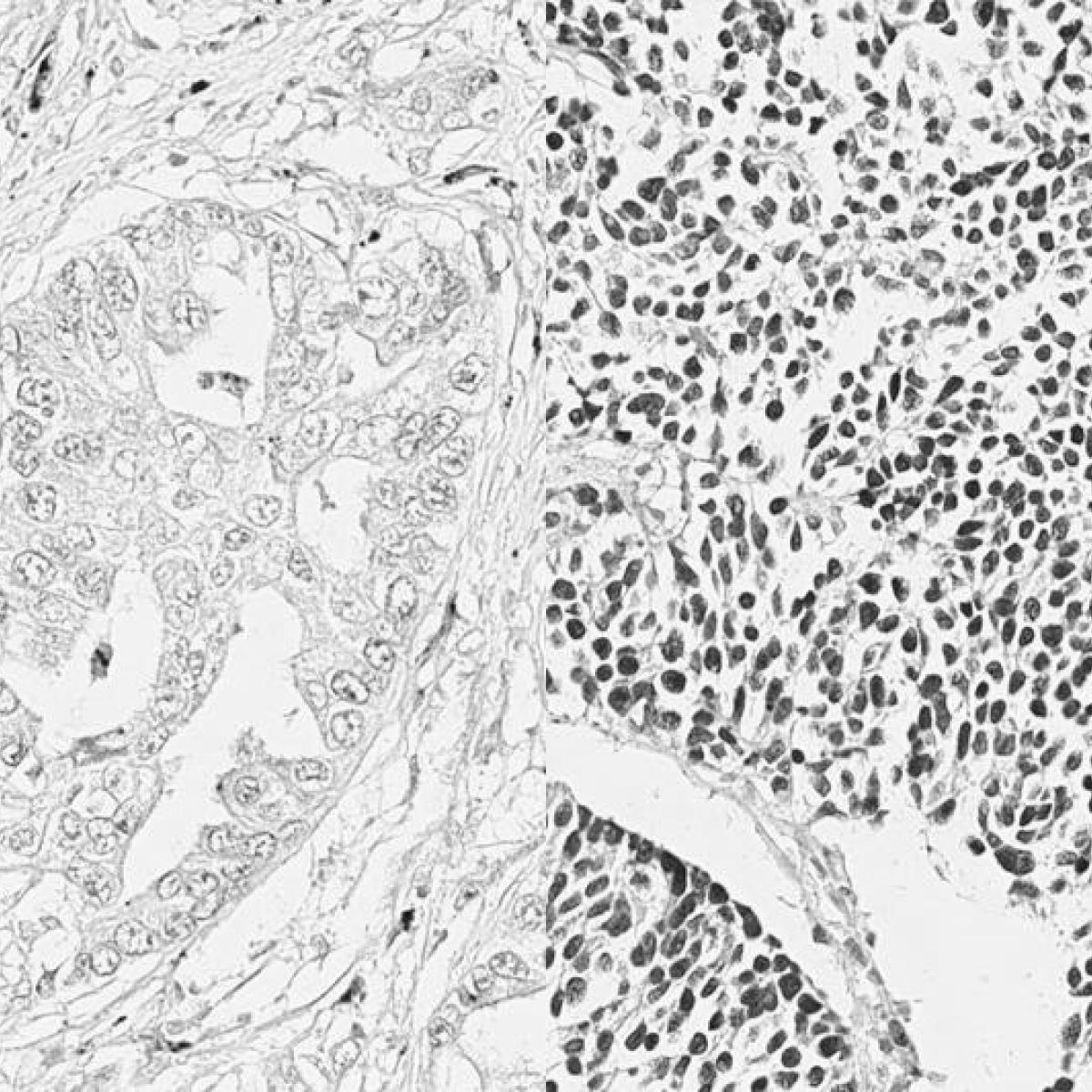
The core provides state-of-the-art, high-resolution technologies for imaging and analyzing the molecular and structural organization of cells and tissue as well as bioengineered materials.
The technologies available in this core include confocal, wide-field fluorescence, live-cell and super-resolution imaging as well as software for image analysis.
The Microscopy Core is organized into three interdependent imaging labs across three locations:
- Center for Health Sciences, Room 66-100E
- Terasaki Life Sciences Building, Room 5031
- Terasaki Life Sciences Building, Room 3128
Training & Scheduling
Training:
Training is required to use the microscopes within the core. To request training, please create a user account in PPMS and request training through the Request tab. If a user does not use the microscopes within three months of completing training, they will be required to attend another training session.
Scheduling:
After completing training, users can schedule time in the Microscopy Core through PPMS. The calendars are self-serve with a 10-day maximum advance scheduling policy.
Equipment
TLSB
Nikon AX R with NSPARC
The AX R NSPARC is an incredibly sensitive and versatile laser scanning confocal capable of fixed or live imaging on an inverted Ti2 stand. The system is equipped with 8 laser lines: 405nm, 445nm, 488nm, 514nm, 561nm, 590nm, 638nm, and 730nm. Additional features include galvo and resonant scanners, spectral tuning via 4 GaAsP detectors, Perfect Focus, and NSPARC array-detector for super-resolution imaging down to ~100nm and outstanding signal-to-noise ratio. Full temperature and CO2 environmental control for live cultures.

Zeiss LSM 880 Confocal with Airyscan
The LSM880 is an inverted laser scanning confocal microscope capable of fixed or live imaging with 7 laser lines: 405nm, 458nm, 488nm, 514nm, 561nm, 594nm, and 633nm; and is equipped with 2 multi-alkali PMT detectors, 1 GaAsP PMT detector and an Airyscan detector for super-resolution imaging down to ~140nm. Full temperature and CO2 environmental control for live cultures. Identical system to CHS 66-100E location.

Zeiss LSM 880 Confocal with 2-photon
The LSM 880 with 2-photon is built on the same inverted platform as our other LSM 880s, but with the added feature of 2-photon imaging with a Coherent Ti-Sapphire laser tunable from 690nm to 1064nm. Ideal for tissue ablation or imaging 2D live cell cultures to semi-thick (~100+ um) sections or use as a standard LSM 880. Equipped with a 32-channel GaAsP spectral detector and 2 multi-alkali PMTs, allowing for lambda scanning and linear unmixing for superior imaging of multiple fluorescent labels.

Zeiss LSM 900 Confocal with Airyscan 2
NEW!
The LSM 900 is an inverted laser scanning confocal microscope designed to accelerate your fluorescence imaging of either fixed or live samples through improved software and detection features. With four laser lines: 405nm, 488nm, 561nm, and 640nm; and three super-sensitive GaAsP detectors, you have the ability to image standard fluorophores with very little laser power. The improved Airyscan 2 detector allows up to 8x faster super-resolution imaging and 6.5x smaller data files compared to the original Airyscan. Automatically identify your sample vessel (e.g. slide or multi-well plate) and tissue sections with the AI sample finder to easily navigate to the region you want to capture.

Zeiss Axioscan 7
NEW!
The Axioscan 7 is a slide scanner that can image up to 100 slides in brightfield or fluorescence with 7 discrete LED lines: 385nm, 430nm, 475nm, 555nm, 590nm, 630nm, and 735nm. Setup automated sample detection with either brightfield or fluorescence to capture all your tissue sections on one or multiple slides. Auto-focus of each sample ensures all your images are in crisp focus with millisecond exposures to minimize photobleaching. Perfect for creating digital archives or for easily imaging whole slides for evaluation without needing to operate a microscope for hours.

IncuCyte SX5 (live-cell analysis)
The IncuCyte SX5 is an automated microscope system housed inside a standard CO2 incubator meant for time-lapse imaging of cell cultures over long periods in 1 or 2+ hour intervals. A variety of vessels can be accommodated from flasks and multiwell plates to chamber slides with the ability to image up to 6 microplates sequentially. Objectives include 4x, 10x, and 20x, with the capability of imaging in phase, green, orange, red and near-IR. Full software package allows for automated data analysis.

TLSB Analysis Workstation 1, Imaris
TLSB Analysis Workstation 1 is loaded with the full Imaris software package, including Clear View-GPU deconvolution and Imaris Stitcher, along with MATLAB. A Puget Systems built workstation with AMD Threadripper Pro 5965WX 3.8GHz 24-core processor, 512GB of RAM, NVIDIA RTX A5000 24GB graphics card, and full M.2 SSD hard drives, this system is capable of converting your most demanding microscopic images into 3D renderings as well as automate analyses of multi-dimensional microscope files.

TLSB Analysis Workstation 2, Elements/Zen
TLSB Analysis workstation 2 is installed with Nikon NIS-Elements Ar for processing and analyzing Nikon microscope files and Zeiss Zen Blue and Black software for processing Zeiss microscope files. An HP Z4 workstation with 6-core Intel Xeon CPU, 128GB RAM, and NVIDIA RTX A4000 16GB graphics card, this system is ideal for basic processing of Zeiss microscope data and advanced processing and analysis of Nikon microscope data.

TLSB Analysis Workstation 3, Zen-Advanced
NEW!
TLSB Analysis workstation 3 is installed with Zeiss Zen 3.12 software for processing and analysis of Zeiss microscope files. An HP Z6 workstation with 8-core Intel Xeon CPU, 192GB RAM, and NVIDIA RTX A5000 24GB graphics card, this system is ideal for analysis of Zeiss microscope data with the Bio Applications module and for processing your Zeiss data, especially large datasets from the Axioscan 7..

CHS
Nikon AX R with NSPARC
The AX R NSPARC is an incredibly sensitive and versatile laser scanning confocal capable of fixed or live imaging on an inverted Ti2 stand. The system is equipped with 4 laser lines: 405nm, 488nm, 561nm, and 638nm. Additional features include galvo and resonant scanners, spectral tuning via 4 GaAsP detectors, Perfect Focus, and NSPARC array-detector for super-resolution imaging down to ~100nm and outstanding signal-to-noise ratio. Full temperature and CO2 environmental control for live cultures.

Zeiss LSM 880 Confocal with Airyscan
The LSM880 is an inverted laser scanning confocal microscope capable of fixed or live imaging with 7 laser lines: 405nm, 458nm, 488nm, 514nm, 561nm, 594nm, and 633nm; and is equipped with 2 multi-alkali PMT detectors, 1 GaAsP PMT detector and an Airyscan detector for super-resolution imaging down to ~140nm. Full temperature and CO2 environmental control for live cultures. Identical system to CHS 66-100E location.

Zeiss Spinning Disk Confocal with Widefield
NEWLY UPGRADED!
The Spinning Disk Confocal is composed of a Yokogawa CSU X1 spinning disk confocal on an inverted Zeiss stand ideal for fast imaging commonly required for live cellular dynamics such as calcium fluorescence. The system is equipped with 6 laser lines: 405nm, 458nm, 488nm, 514nm, 561nm, and 633nm, and Hamamatsu Orca-Flash 4.0 v3 sCMOS camera with a maximum ~80 fps full FOV capture rate. Widefield imaging can be accomplished with the attached AxioCam 702 and Colibri 7 light source for imaging up to ~120 fps full FOV with 7 LED lines: 385nm, 430nm, 475nm, 555nm, 590nm, 630nm, and 735nm. Full temperature and CO2 environmental control for live cultures.

Zeiss LSM 700 Confocal
The LSM 700 is an inverted laser scanning confocal microscope capable of fixed or live imaging with 4 laser lines: 405nm, 488nm, 555nm, and 639nm; and 2 multi-alkali PMT detectors. This system is ideal for standard 4 color imaging of common fluorophores such as DAPI, GFP, RFP, and far-red (e.g. Cy5).

CHS Analysis Workstation 1, Imaris
CHS Analysis Workstation 1 is loaded with the full Imaris software package including Clear View-GPU deconvolution and Imaris Stitcher, along with MATLAB. A Puget Systems built workstation with AMD Threadripper Pro 5975WX 3.6GHz 32-core processor, 512GB of RAM, NVIDIA RTX A4000 16GB graphics card, and full M.2 SSD hard drives, this system is capable of converting your most demanding microscopic images into 3D renderings as well as automate analyses of multi-dimensional microscope files.
CHS Analysis Workstation 2, Elements/Zen
UPGRADED!
CHS Analysis Workstation 2 is installed with Nikon NIS-Elements Ar for processing and analyzing Nikon microscope files and Zeiss Zen 3.12 software for processing Zeiss microscope files. An HP Z4 workstation with 6-core Intel Xeon CPU, 128GB RAM, and NVIDIA RTX A4000 20GB graphics card, this system is ideal for basic processing of Zeiss microscope data and advanced processing and analysis of Nikon microscope data.
Pricing
| Product/Service | UCLA Rate | External Non-Profit Rate | External Rate |
| LSM 900 and AX Confocal - Peak | $43.41 | $60.34 | $108.62 |
| LSM 900 and AX Confocal - Off Peak | $29.03 | $40.36 | $72.64 |
| LSM 880 - Peak | $35.79 | $49.75 | $89.55 |
| LSM 880 - Off Peak | $24.49 | $34.04 | $61.28 |
| LSM 700 and Spinning Disk - Peak | $30.46 | $42.34 | $76.21 |
| LSM 700 and Spinning Disk - Off Peak | $21.83 | $30.34 | $54.61 |
| Axioscan 7 - Peak | $24.07 | $33.46 | $60.22 |
| Axioscan 7 - Overnight (6PM – 9AM) | $162.81 | $226.31 | $407.35 |
| IncuCyte SX5 | $2.69 | $3.74 | $6.73 |
| Imaris Workstation | $9.06 | $12.60 | $22.67 |
| Training | $92.71 | $128.87 | $231.96 |
*Please note: Peak hours are 9AM to 6PM Monday to Friday. Off-peak hours are 6PM to 9AM Monday to Friday, all day weekends and holidays.
FAQs
The Center for Health Sciences Microscopy Core is located in the South Tower of CHS on the 6th floor and requires UCLA David Geffen School of Medicine badge access for entry.
The Terasaki Life Sciences Building Microscopy Cores are located in the West Tower of TLSB on the 3rd and 5th floors. Access is restricted. Feel free to contact Ken Yamauchi at KenY@ucla.edu to arrange a tour of the core facilities.
For help identifying which microscope would suit your needs, contact Ken Yamauchi at KenY@ucla.edu. The core has everything from a simple-to-use confocal (LSM700) to wide-field epi-fluorescence or color imaging (Imager with Apotome) to deeper tissue imaging (2-photon).
Most systems have a range of objectives from 5x, 10x, 20x, 40x, 63x and 100x. Contact Ken Yamauchi at KenY@ucla.edu for more details.
- Create an account:
- Navigate to our online scheduling software PPMS at and request to create a user account.
- Request training:
- Once your account is approved, log in to PPMS with your newly created credentials and click on “request” in the top menu to request the microscope you would like to be trained to use.
- Sign up for training:
- Once your training request is submitted, you will enter a queue and will be contacted by email for the next available training session. Trainings are typically held twice monthly and are in a group setting.
Yes, contact Ken Yamauchi at KenY@ucla.edu; he will be happy to assist you. The more details you provide, the better he will be able to help with your imaging.
Unfortunately, only users who have been trained and approved by core staff are allowed to use the Microscopy Core systems.
Please include the UCLA Broad Stem Cell Research Center Microscopy Core in your acknowledgements section.