April Pyle, Ph.D.
- Professor and Vice Chair, Microbiology, Immunology and Molecular Genetics
- George and Nouhad Ayoub Centennial Chair, Life Sciences Innovation

April Pyle, Ph.D., studies how skeletal muscle stem cells arise in human development and investigates the differences between skeletal muscle progenitor and muscle stem cells. She aims to apply these insights to develop gene editing and stem cell-based treatments for Duchenne muscular dystrophy and other neuromuscular diseases.
Pyle takes a multidisciplinary approach to studying muscle stem cells, human pluripotent stem cells and their differentiation processes to advance their use in neuromuscular disease modeling and the development of regenerative medicine and gene editing approaches. Her research encompasses both basic aspects of stem cell biology and development as well as more translational aspects of muscle stem cell research that leverage stem cells and CRISPR gene-editing technologies toward novel therapies.
In collaboration with center member Melissa J. Spencer, Ph.D., Pyle is developing methods to deliver CRISPR/Cas9 components directly to satellite cells for in vivo gene editing, as well as integrating gene editing with ex vivo stem cells derived from human pluripotent stem cells to restore muscle function in individuals with Duchenne muscular dystrophy. She and Spencer co-founded biotechnology start-up, MyoGene Bio, to develop a therapeutic CRISPR/Cas9 platform to restore the reading frame of the Duchenne muscular dystrophy gene by directly deleting exons 45-55 in skeletal muscle.
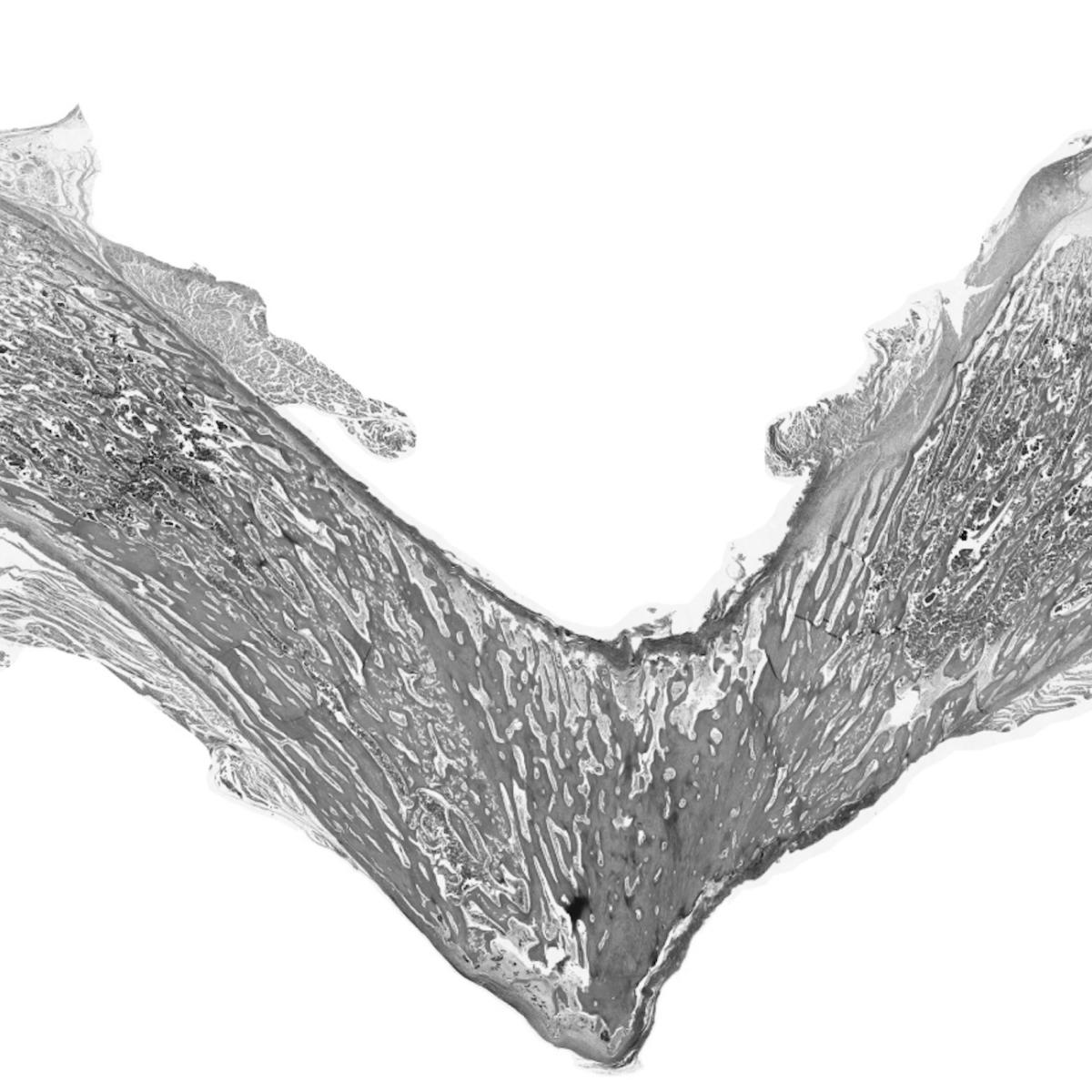
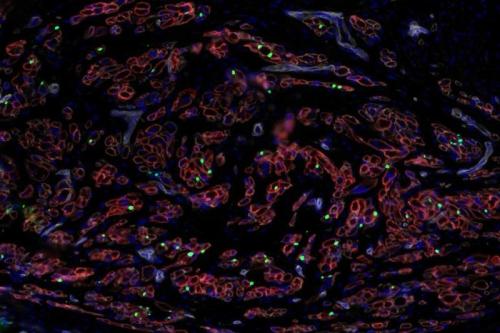
Pyle’s extensive study of the biological underpinnings of Duchenne muscular dystrophy has led to several significant breakthroughs. She developed a first-of-its-kind roadmap of human skeletal muscle formation and devised strategies to generate skeletal muscle cells and somites, the cells that give rise to skeletal muscles, bones and cartilage during development, from human pluripotent stem cells. Each of these discoveries represents a major step toward the development of a cell replacement therapy for Duchenne.
"A story like MyoGene’s would only be feasible in a few places. The environment at UCLA, and in particular, the Broad Stem Cell Research Center, has really enabled us to go from a basic, innovative idea to these early preclinical studies. There are also pipelines and mentors in place here to build the partnerships necessary to move early discoveries toward the clinic."
- Professor and Vice Chair, Microbiology, Immunology and Molecular Genetics
Research Projects
- Developing CRISPR A gene editing technology that enables scientists to remove, add or alter DNA at precise locations in the genome to prevent, treat or cure disease. One component acts as a navigation system that can be programmed to seek out a particular DNA sequence and the other component acts as a pair of "molecular scissors" that can cut two strands of DNA at that location to enable gene modification. CRISPR A gene editing technology that enables scientists to remove, add or alter DNA at precise locations in the genome to prevent, treat or cure disease. One component acts as a navigation system that can be programmed to seek out a particular DNA sequence and the other component acts as a pair of "molecular scissors" that can cut two strands of DNA at that location to enable gene modification./Cas9 gene editing A type of gene therapy that works by delivering genetic material that can directly edit pieces of DNA within a cell. This changes the instructions the DNA encodes for, which ultimately results in an increase or decrease in the production of a certain protein and the restoration of proper cell function. gene editing A type of gene therapy that works by delivering genetic material that can directly edit pieces of DNA within a cell. This changes the instructions the DNA encodes for, which ultimately results in an increase or decrease in the production of a certain protein and the restoration of proper cell function. therapies to correct the mutation that causes Duchenne muscular dystrophy
- Developing tailored engineering platforms to enhance the ability to reprogram, expand and/or differentiate human pluripotent stem cells Stem cells that can undergo self-renewal and differentiation to become any cell type found in the body. The two major types used in research are embryonic stem cells and induced pluripotent stem cells. pluripotent stem cells Stem cells that can undergo self-renewal and differentiation to become any cell type found in the body. The two major types used in research are embryonic stem cells and induced pluripotent stem cells. to generate robust and stable differentiated progenitors
- Exploring approaches to increase the efficiency of AAV vector targeting to enable treatment strategies that correct disease-causing genetic defects in muscle satellite cells in vivo A process, procedure or study performed on or in a living organism. in vivo A process, procedure or study performed on or in a living organism.
- Identifying the most engraftable cell type, signaling factors and niche components to increase the potential of cell-based therapies using stem cell-derived skeletal muscle progenitor cells Descendants of stem cells that can further differentiate to produce one or more specialized cell types. They are more limited than pluripotent stem cells in that they cannot self-renew indefinitely and can only produce a limited range of specific cell types. For example, neural progenitor cells can only produce neurons. progenitor cells Descendants of stem cells that can further differentiate to produce one or more specialized cell types. They are more limited than pluripotent stem cells in that they cannot self-renew indefinitely and can only produce a limited range of specific cell types. For example, neural progenitor cells can only produce neurons.
- Developing stem cell-based models of motor neuron diseases like ALS to study disease progression and develop therapies to extend the lives of patients with these conditions
- Pinpointing the different stages of human developmental myogenesis, the process by which muscle tissue is formed and developed
-
Post-doctoral Fellowship
- Johns Hopkins University, 2006
Degree
- Ph.D., Cell and Molecular Biology, University of Tennessee, 2002
-