Samantha J. Butler, Ph.D.
- Professor, Neurobiology
- Vice Chair of Community, Neurobiology
Samantha Butler, Ph.D., studies how the nervous system — the complex network made up of the brain, spinal cord and nerves that run through the body — connects and grows during fetal development. Her research could inform the development of drug and stem cell-based therapies that help people with spinal cord injuries and nerve damage regain sensation.
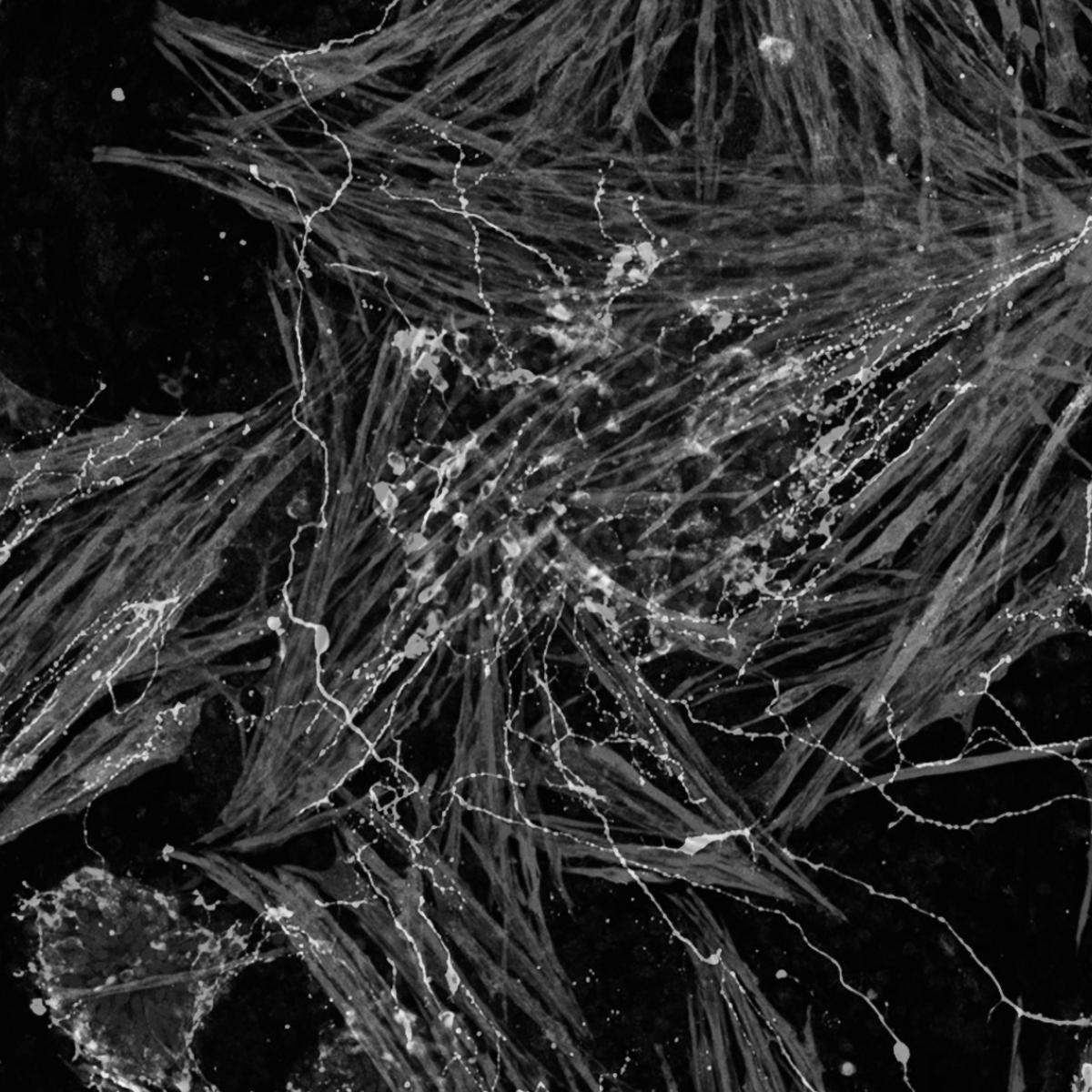
The extraordinarily diverse functions of the nervous system, from cognition to movement, are possible because neurons are assembled into precisely ordered networks that permit them to rapidly and accurately communicate with their synaptic targets. For nearly two decades, Butler has been at the forefront of understanding the mechanisms that establish and regenerate this neural circuitry.
By pursuing a greater understanding of how the nervous system is established, Butler hopes to determine how this process can be recreated or co-opted to regenerate diseased or damaged nerves and nerve circuits. Her work overturned a long-standing paradigm about how axons — thread-like projections that connect cells in the nervous system — grow during embryonic development. Butler discovered that neural progenitors organize axon growth by producing a pathway of a protein called netrin1 that leads them through their local environment.
This insight into axon growth could enable scientists to use netrin1 to enhance and accelerate recovery in patients with serious nerve damage, such as veterans with combat injuries. Without intervention, these patients face lengthy and painful recovery times, and can lose both movement and sensation in the injured limb due to the gradual loss of nerve cells after injury.
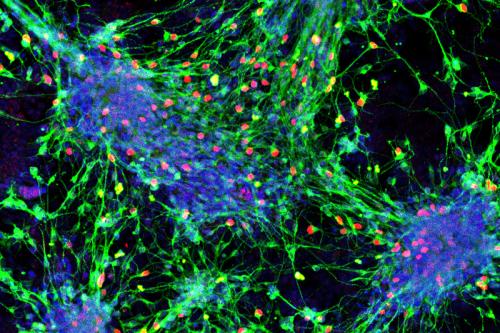
Butler recently created a method for producing all types of sensory interneurons from mouse stem cells and is now working to replicate this work in human cells. If successful, this could be a key step toward the development of stem cell-based therapies to regenerate or repair damaged or abnormal sensory neurons, which can result from physical trauma or injury, chemotherapy, autism, aging or other diseases.
Research Projects
- Developing techniques to produce all subtypes of sensory interneurons from human stem cells Cells that have the ability to differentiate into multiple types of cells and make an unlimited number of copies of themselves. stem cells Cells that have the ability to differentiate into multiple types of cells and make an unlimited number of copies of themselves., a critical step towards the development of cell therapies that restore sensation in people with spinal cord injuries
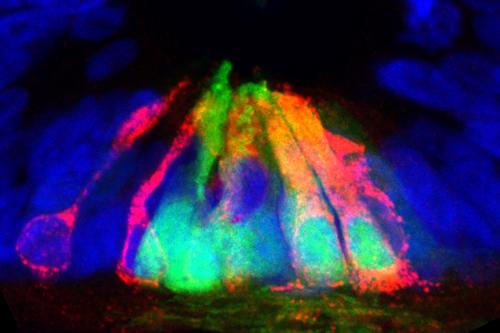
- Understanding the mechanisms by which dorsal sensory interneurons arise in the spinal cord
- Determining how the rate of nerve growth is regulated in development and regeneration to inform the creation of approaches to accelerate this process, resulting in faster recovery times for patients with nerve damage
- Understanding the mechanisms of axon guidance in the spinal cord, which could lead to methods to replicate or control how axons regenerate
-
Post-doctoral Fellowship
- Developmental Neurobiology, Columbia University, 2003
Degree
- Ph.D., Molecular Biology, Princeton University, 1996
-