The fundamentals of gene regulation: Study challenges prior understanding of X chromosome inactivation
Kathrin Plath is fascinated by the fundamental processes that regulate pluripotent stem cells and allow them to turn into any cell type in the body. Her research advances understanding of the epigenetic regulation of gene expression. Insights gained from these efforts can help fellow scientists use the most pristine human stem cells in their research, improving models of both disease and early human development.
One area of particular interest in the Plath laboratory is X chromosome inactivation, a process in which one of the two X chromosomes in female cells is “turned off” to balance gene expression.
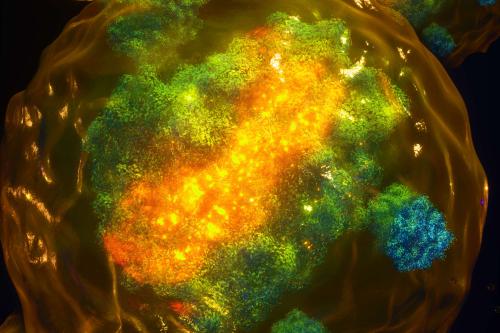
In a new study published in the journal Cell, Plath challenges longstanding beliefs about the role of the long non-coding RNA XIST finding that it not only binds to the X chromosome, but also to autosomes, the non-sex chromosomes that carry most of the body’s genetic information. As on the X chromosome, XIST can also lead to lower gene expression on autosomes. This finding could change the way we think about and develop treatments for female-specific diseases.
Here, Plath, a professor of biological chemistry and member of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA, breaks down these novel findings. She is joined by the study’s co-first author, Iris Dror, a post-doctoral fellow in Plath’s laboratory, to share how their findings could lay the groundwork for new therapies for female-specific diseases.
What motivated your team to investigate XIST’s role in gene regulation?
X chromosome inactivation is a fundamental epigenetic process that happens in early development of mammalian females. This process silences a whole X chromosome; without it, development stalls. X-inactivation happens very early — when stem cells are still in a pluripotent state. Understanding how XIST RNA can recruit various regulatory proteins to the X chromosome to induce silencing and large-scale remodeling of an entire chromosome is fascinating.
Females have two X chromosome whereas males only have one, and X-inactivation equalizes the genetic playing field. One of those X chromosomes in females needs to be silenced, or made inactive, to enable normal development and avoid potential adverse health effects. Given this importance for proper development, studying how XIST induces X-inactivation enriches our knowledge of the fundamental processes that shape life from its earliest stages.
X-inactivation involves epigenetic modifications, such as DNA methylation and histone modifications. Studying these epigenetic changes on the X chromosome provides insights into the broader field of epigenetics, helping researchers understand how genes are regulated and how these regulatory mechanisms contribute to normal development and disease.
There are thousands of other non-coding RNAs in the nucleus and many of these likely have important functions. XIST has become an ideal model to investigate how RNAs act in the nucleus and can alter gene expression.
What are the key findings of this paper?
There are three fundamental things we always thought about XIST: that it is only expressed on one X chromosome in female cells; that it only spreads across the X chromosome from which it is expressed (i.e. it acts “in cis”) to silence genes; and that its main role is to balance gene expression between females and males.
Our paper challenges these essential paradigms.
It was recently discovered that a unique form of X inactivation takes place during early embryonic development in humans, where XIST is expressed from both X chromosomes. We found that during this stage, XIST spreads beyond the X chromosome and onto autosomes. And not only is XIST moving to autosomes, it is also regulating the genes it targets on autosomes and reducing their expression. Thus, XIST can also act “in trans,” or beyond the chromosome from which it is expressed. Because XIST is unique to females, the action of XIST on autosomal genes causes differences in how genes are expressed between the sexes. Thus, while XIST ensures comparable gene expression levels between females and males on the X-chromosome, it introduces differences on autosomes.
Initially, we thought the finding of autosomal XIST action is specific only to early human development. However, we realized that the autosomal targeting of XIST is associated with a dispersed pattern of XIST in the nucleus. This XIST dispersal is observed in early human development and resolves into a compact localization pattern later. Intriguingly, we also observed a similar dispersed localization of XIST during a brief window of early mouse development and showed that it correlates with spreading of XIST to autosomes, as seen in human development. Therefore, this new “trans” function of XIST likely happens in all mammals.
How does this research impact our understanding of X chromosome inactivation?
It’s especially interesting to learn that XIST does more than just function on the X chromosome, which opens a completely new view that will change textbook knowledge.
It also creates many more questions: How does XIST find its targets on autosomes? Why does XIST sometimes only work on the X chromosome and in other instances on the X chromosome as well as autosomes? How does the regulation of genes on autosomes alter early development in females?
Even though XIST is one of the most well-studied long non-coding RNAs in the nucleus, the new autosomal function of XIST needs further exploration. We have years if not decades of research ahead of us.
Could you explain why the discovery that XIST acts on autosomes is significant?
The autosomal action of XIST we discovered only occurs in female cells in early development and not in males. We speculate that this might allow female cells to slightly change the timeline of early development, which may give XIST enough time to silence all genes on the X chromosome.
Showing that the dispersal of XIST in the nucleus correlates with its spreading to autosomes in early human and mouse development provides another important new research dimension. There are a lot of new publications showing that the dispersed configuration of XIST is also found in cancer cells and in immune cells. In these cases, it’s also likely that XIST plays a role in regulating autosomal regions and contributes to cancer development or autoimmunity in females. We will further explore this idea in the future and investigate what the XIST dispersal in these cells means.
The XIST dispersal in human and mouse development happens before hormonal expression starts in the embryo. It could explain some of the early changes in our gene expression between sexes that are not hormone dependent.
How might these findings impact human health or inform the development of therapies in the future?
XIST’s unique properties are already being explored as a therapeutic tool to treat some diseases. A key example would be in Down syndrome where scientists have used pluripotent stem cells to demonstrate that XIST can silence the extra chromosome found in individuals with the disorder. If we improve our understanding of XIST’s various functions, we can harness it for additional epigenetic therapeutics.
In addition, female-specific cancers, or even autoimmune diseases — which are more common in females — may be at least in some part dependent on XIST’s spreading to autosomes. This research could improve our understanding of the regulation and development of female-specific diseases and potentially inspire new treatments.
What else would you like readers to know about this paper?
It’s important to emphasize the collaborative nature of this research; without our collaborators this work would not have been possible. Iris played a pivotal role in coordinating and synthesizing the efforts of a diverse group of scientists, to seamlessly integrate computational analyses with wet bench experiments, resulting in a comprehensive and convincing narrative.
Authors
Dror’s co-first author is Tsotne Chitiashvili, a former graduate student in the Plath lab. Other UCLA authors include Shawn Y.X. Tan, Clara T. Cano, Anna Sahakyan, Yolanda Markaki, Constantinos Chronis, Amanda J. Collier, Weixian Deng, Yu Sun, Anna Afasizheva, Jarrett Miller, Wen Xiao and Douglas L. Black. Guohao Liang and Fangyuan Ding of UC Irvine contributed to the study.
This conversation has been edited for length and clarity.
Notes
This study was funded by the National Institutes of Health, the California Institute for Regenerative Medicine, the National Institutes of Health National Center for Advancing Translational Science-supported UCLA Clinical and Translational Science Institute, both an innovation award and a training fellowship from the UCLA Broad Stem Cell Research Center, the Howard Hughes Medical Institute, the Iris Cantor-UCLA Women’s Health Center, the UCLA David Geffen School of Medicine and the UCLA Jonsson Comprehensive Cancer Center.

