Could the ‘central dogma’ of biology be misleading bioengineers?
Key takeaways/Summary
- Mesenchymal stem cells, found in bone marrow, secrete therapeutic proteins that could potentially help regenerate damaged tissue.
- A UCLA study examining these cells challenges the conventional understanding of which genetic instructions prompt the release of these therapeutic proteins.
- The findings could help advance both regenerative medicine research and the laboratory production of biologic treatments already in use.
Today, medicines based on antibodies — proteins that fight infection and disease — are prescribed for everything from cancer to COVID-19 to high cholesterol. The antibody drugs are supplied by genetically engineered cells that function as tiny protein-producing factories in the laboratory.
Meanwhile, researchers have been targeting cancer, injuries to internal organs and a host of other ailments with new strategies in which similarly engineered cells are implanted directly into patients.
These biotechnology applications rely on the principle that altering a cell’s DNA to produce more of the genetic instructions for making a given protein will cause the cell to release more of that protein.
A new UCLA study suggests that — at least in one type of stem cell — the principle doesn’t necessarily hold true.
The researchers examined mesenchymal stem cells, which reside in bone marrow and can self-renew or develop into bone, fat or muscle cells. Mesenchymal cells secrete a protein growth factor called VEGF-A, which plays a role in regenerating blood vessels and which scientists believe may have the potential to repair damage from heart attacks, kidney injuries, arterial disease in limbs and other conditions.
When the researchers compared the amount of VEGF-A that each mesenchymal cell released with the expression of genes in the same cell that code for VEGF-A, the results were surprising: Gene expression correlated only weakly with the actual secretion of the growth factor.
The scientists identified other genes better correlating with growth factor secretion, including one that codes for a protein found on the surface of some stem cells. Isolating stem cells with that protein on their surface, the team cultivated a population that secreted VEGF-A prolifically and kept doing so days later.
The findings, published today in Nature Nanotechnology, suggest that a fundamental assumption in biology and biotechnology may be up for reconsideration, said co-corresponding author Dino Di Carlo, the Armond and Elena Hairapetian Professor of Engineering and Medicine at the UCLA Samueli School of Engineering.
“The central dogma has been, you have instructions in the DNA, they’re transcribed to RNA, and then the RNA is translated into protein,” said Di Carlo, who is also a member of UCLA’s California NanoSystems Institute and Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research. “Based on this, many scientists assumed that if you had more RNA, you’d have more protein, and then more protein released from the cell. We questioned that assumption.
“It seems we can’t assume that if a gene is expressed at higher levels, there will be higher secretion of the corresponding protein. We found a clear example where that doesn’t happen, and it opens up a lot of new questions.”
The results could help make the manufacturing of antibody-based treatments more efficient and define new cellular treatments that would be more effective. Knowing the right genetic switches to flip could enable the engineering or selection of extraordinarily productive cells for making or delivering therapies.
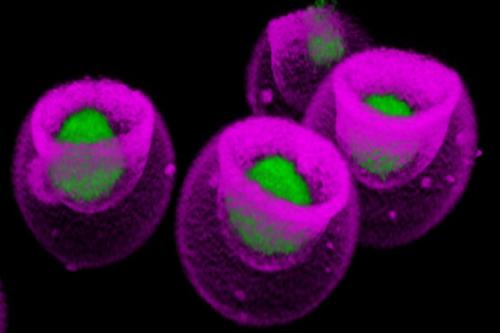
The UCLA study was conducted using standard lab equipment augmented with a technology invented by Di Carlo and his colleagues: nanovials, microscopic bowl-shaped hydrogel containers, each of which captures a single cell and its secretions. Leveraging a new nanovial-enabled analytic method, the scientists were able to connect the amount of VEGF-A released by each one of 10,000 mesenchymal stem cells to an atlas mapping tens of thousands of genes expressed by that same cell.
“The ability to link protein secretion to gene expression on the single-cell level holds great promise for the fields of life science research and therapeutic development,” said Kathrin Plath, a UCLA professor of biological chemistry, a member of the Broad Stem Cell Research Center and a co-corresponding author of the study. “Without it, we couldn't have arrived at the unexpected results we found in this study. Now we have an exciting opportunity to learn new things about the mechanisms underpinning the basic processes of life and use what we learn to advance human health.”
While activation of the genetic instructions for VEGF-A displayed little correlation with release of the protein, the researchers identified a cluster of 153 genes with strong links to VEGF-A secretion. Many of them are known for their function in blood vessel development and wound healing; for others, their function is currently unknown.
One of the top matches encodes a cell-surface protein, IL13RA2, whose purpose is poorly understood. Its exterior location made it simpler for the scientists to use it as a marker and separate those cells from the others. Cells with IL13RA2 showed 30% more VEGF-A secretion than cells that lacked the marker.
In a similar experiment, the researchers kept the separated cells in culture for six days. At the end of that time, cells with the marker secreted 60% more VEGF-A compared to cells without it.
Although therapies based on mesenchymal stem cells have shown promise in laboratory studies, clinical trials with human participants have shown many of these new options to be safe but not effective. The ability to sort for high VEGF-A secreters using IL13RA2 may help turn that tide.
“Identifying a subpopulation that produces more, and markers associated with that population, means you can separate them out very easily,” Di Carlo said. “A very pure population of cells that’s going to produce high levels of your therapeutic protein should make a better therapy.”
Notes
Nanovials are available commercially from Partillion Bioscience, a company co-founded by Di Carlo that started up at the CNSI’s on-campus incubator, Magnify.
The first author of the study is Shreya Udani, who earned a doctorate from UCLA in 2023. Other co-authors, all affiliated with UCLA, are staff scientist Justin Langerman; Doyeon Koo, who earned a doctorate in 2023; graduate students Sevana Baghdasarian and Citradewi Soemardy; undergraduate Brian Cheng; Simran Kang, who earned a bachelor’s degree in 2023; and Joseph de Rutte, who earned a doctorate in 2020 and is a co-founder and CEO of Partillion.
The study was supported by the National Institutes of Health and a Stem Cell Nanomedicine Planning Award funded jointly by the CNSI and the Broad Stem Cell Research Center.
