Natural compound protects against Zika virus infection and microcephaly
FINDINGS
New UCLA research could lead to the development of a drug that combats several mosquito-borne viruses, including Zika virus. The research reveals that an enzyme produced naturally by the immune system protects animals against Zika virus infection and the neurological damage linked to the virus. The enzyme, called 25-hydroxycholestrol or 25HC, can be manufactured to create a compound that works against a broad range of viruses. The research was led by senior author Genhong Cheng, professor of microbiology, immunology and molecular genetics and a member of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA.
BACKGROUND
Zika virus was not commonly seen in humans before 2007. However, the virus became widely recognized due to large outbreaks in the Caribbean and South America in 2015 that were associated with unusually high incidences of severe birth defects in children born to Zika-infected mothers. Zika virus can be sexually transmitted, but it is primarily spread by a type of tropical mosquito and is closely related to the mosquito-borne dengue and yellow fever viruses.
Most people infected with the Zika virus either don’t get sick or experience mild symptoms; a very small number of people develop temporary muscle weakness or even paralysis. Zika virus has become a public health threat due to its rapid spread throughout the world and link to severe birth defects including microcephaly, in which babies are born with abnormally small heads resulting from underlying brain damage. There are no approved antiviral drugs or vaccines to combat Zika virus and no treatments for the associated microcephaly in babies.
Previous preclinical research published by Cheng and his collaborators in 2013 showed that the body produces 25HC naturally to aid the immune system as it fights viruses. While the natural production of 25HC is typically not strong enough to combat many aggressive viruses, the 2013 research showed that administration of a synthetically produced version of 25HC protects cells from infection by a range of viruses such as hepatitis C, Ebola and human immunodeficiency virus, or HIV. However, 25HC’s effect on the Zika virus had never been tested.
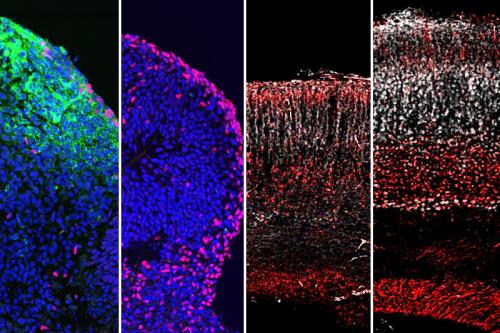
METHOD
The team found that 25HC reduces the amount of Zika, dengue and yellow fever viruses in infected cells in a petri dish. The research team then tested 25HC’s effect on Zika virus by administering synthetically produced 25HC to animals with the Zika virus. They found that 25HC significantly suppressed Zika virus infection in the animals and reduced brain damage in fetal mice. 25HC was also tested in mini brain “organoids” which are simplified, three-dimensional versions of the human brain produced in petri dishes using human stem cells. These organoids mimic human-specific characteristics of brain growth that cannot be tested in animals. Results revealed that 25HC also blocks Zika virus infection and preserves human brain cell formation.
IMPACT
The new research highlights the potential use of 25HC to combat Zika virus infection and prevent its devastating outcomes, such as microcephaly. The research team will further study whether 25HC can be modified to be even more effective against Zika and other mosquito-borne viruses.
AUTHORS
In addition to Cheng, the co-first authors are Chunfeng Li, Yong-Qiang Deng, Shuo Wang, Feng Ma, Saba Roghiyh Aliyari and Xing-Yao Huang. Co-corresponding authors include Bennett Novitch, Zhiheng Xu, and Cheng-Feng Qin. Additional co-authors and author institutional affiliations can be found in the manuscript.
JOURNAL
The study was published in the journal Immunity.
FUNDING
The study was funded by grants from the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (2016-I2M-1-005), the National Science and Technology Major Project for Significant New Drugs Innovation and Development (2015ZX09102023), the National Natural Science Foundation of China (91542201, 81590765, 81522025, 81661130162, 31430037, 31500145 and 31670883), the National Institutes of Health (R01 R01AI069120, AI056154, NS089817 and AI078389), the Peking Union Medical College Youth Fund (3332016125), the Ministry of Science and Technology of the People’s Republic of China (2016YFD0500304, 2014CB942801 and 2012YQ03026006), the State Key Laboratory of Pathogen and Biosecurity (SKLPBS1601), the Innovative Research Group (81621005), the Newton Advanced Fellowship from the United Kingdom Academy of Medical Sciences, the Shanghai brain-intelligence project from the Shanghai Science and Technology Committee (16JC1420500), the Beijing brain project (Z161100002616004), the California Institute for Regenerative Medicine (DISC1-08819), and both a research award and a training fellowship from the UCLA Broad Stem Cell Research Center.