
Decoding the mysteries of ovaries
“Sinthia, can you please grab the little ovaries,” Amander Clark, PhD, calls out to a lab technician in a long blue coat. The “little ovaries” aren’t genuine ovaries, of course — those would be difficult to grab, given their location inside the mammalian body — but a small tray of organ models engineered from different types of mouse stem and progenitor cells. By providing them with nutrients and a collagen-coated scaffolding on which to grow, Dr. Clark, a stem cell scientist and developmental biologist who is director of the UCLA Center for Reproductive Science, Health and Education and professor of molecular, cell and developmental biology in the UCLA College Division of Life Sciences, coaxes them to undergo a process of self-organization into a model of an organ that will eventually be capable of the ovary’s chief function: production of eggs and hormones.
“We’re trying to create folliculogenesis [creation of an egg], hormone production and ovulation in culture,” Dr. Clark says. This complex process takes 23 days. It begins with the creation of germ cells from stem cells, which are combined with somatic progenitor cells derived from fetal mouse ovarian tissue. Then, Dr. Clark says, “The magic happens: They self-assemble and form what we call ‘reconstituted ovaries.’” There are several cohorts of reconstituted ovaries underway, some of them eight days into the developmental process, some at 21 days. They are growing in wells in covered plastic dishes, labeled with the date and lot number. Under the right circumstances, they will form follicles containing oocytes, which, once they mature, become ova — i.e., eggs.
They are barely visible to the naked eye, but under a microscope, Dr. Clark watches the eight-day-old ovary at work. A bit like a half-painted landscape, in which one can see the forms starting to take shape, the immature reconstituted ovary is a work in progress. Dr. Clark points to the stem-cell derived germ cells that have been marked with blue fluorescence. “You can see some of them down here, these little circles — they’re tiny, tiny oocytes that are starting to form.” In the same reconstituted ovary, she grows both stem-cell derived oocytes and oocytes carried through with the ovarian somatic progenitors, allowing her to compare how the lab-grown oocytes compare to ones that had already started to develop in the ovary. That’s “the beautiful thing about this system,” she says. “What we want to see is how similar are the blue ones, the stem-cell derived ones, within the same organ to the wild type ones?”
The 21-day-old reconstituted ovaries have formed follicles, each of which contains a single oocyte, as well as granulosa cells, which release hormones, including estrogen, progesterone and testosterone. The estrogen released in the initial phase of the ovulatory cycle tells the oocyte to keep growing. Eventually, a clutch of these immature follicles will activate, a process caused by mechanisms that, Dr. Clark says, “are not well understood.” In humans, one follicle becomes the dominant one, released by the ovary into the fallopian tube during ovulation. By studying every step of this process under a microscope, Dr. Clark and her colleagues hope to better understand this basic reproductive function. Her insights could help people increase their reproductive success, particularly by improving costly and too-often unsuccessful fertility treatments.
OVARIES PLAY AN ESSENTIAL ROLE IN THE PERPETUATION OF OUR SPECIES, BUT THEY DON’T ALWAYS FUNCTION AS THEY SHOULD. THE WORLD HEALTH ORGANIZATION RECENTLY ESTIMATED THAT INFERTILITY AFFECTS ONE-IN-SIX COUPLES GLOBALLY.
Ovaries play an essential role in the perpetuation of our species, but they don’t always function as they should. The World Health Organization recently estimated that infertility affects one-in-six couples globally. The common reproductive disorder polycystic ovary syndrome is associated with metabolic disease, mood disorders and difficulty getting pregnant. The ovaries also play host to one of the deadliest known cancers, which has proven especially difficult to screen for and treat. Armed with a wide range of methodologies, such as stem-cell-derived ovaries, genetic databases and animal models, researchers and clinicians at UCLA are unlocking the secrets of this mysterious, complex and powerful organ.
“Ovaries are fascinating organs. I always say they look like little brains,” says Beth Karlan, MD (FEL ’89), the Nancy Marks Endowed Chair in Women’s Health Research in the David Geffen School of Medicine at UCLA and director of cancer population genetics in the UCLA Health Jonsson Comprehensive Cancer Center. “They are not only responsible for the ongoing of our race and society as humans, but also for all the hormones that lead women through life and longevity, whether it’s through puberty, through menopause or beyond.”
FOR PEOPLE HOPING TO GET PREGNANT, one of the most important factors is “oocyte competency,” that is, the oocyte’s capacity to mature, fertilize, implant and grow into a healthy baby. About one-third of infertility cases are attributed to female-factor infertility (another third is male-factor, and the remaining third of cases are unexplained or related to multiple factors). Today, there is still no reliable way to assess oocyte competency before conception.
Moreover, humans are born with all the oocytes they’ll ever have, a number estimated at 700,000-to-1 million. That number declines with age until menopause, and the quality of the eggs deteriorates, too. As women age, the oocyte maturation process is more error-prone, resulting in more chromosomal abnormalities that lower the chances of pregnancy. “What’s really missing in this field is non-invasive ways to test oocyte competence,” Dr. Clark says. “If a woman is going through IVF, and there are a lot of eggs retrieved at once, how do we tell which egg is competent?”
Without insight into the health of the egg, fertility doctors are left with no option but to fertilize all the eggs retrieved and then choose the best embryo based on its appearance or the results of genetic testing, a practice that can result in an excess of embryos, unsuccessful embryo transfers or both. Similarly, a patient undergoing oocyte cryopreservation (also known as “egg freezing”) cannot know if those eggs are healthy until she chooses to fertilize them, at which point the patient may be significantly older or no longer ovulating.
Lab-grown human eggs are still, in all likelihood, in the distant future. But with her reconstituted ovaries, Dr. Clark can study minute aspects of oocyte development in order to pinpoint where the process goes awry. Although they are not exact replicas of a human ovary — for starters, they are made from mice cells — they are built from cells similar to those that make up a human ovary. Her team can set up about 10-to-12 ovaries every two weeks, and each one will typically yield about 50 oocytes, giving Dr. Clark many data points to study.
This is fortunate, as there are many aspects of oocyte development that remain shrouded in mystery. How, for example, does energy metabolism function to help or hinder the growth of an egg? It is known that fertilized oocytes must use a lot of mitochondria, which provide the energy that powers a cell’s biochemical reactions, in order to be able to support early embryo development. Another key part of oocyte development is germinal vesicle breakdown, in which the oocyte’s nucleus dissolves during the luteinizing hormone surge in order to resume meiosis, the special type of cell division that creates sperm and eggs. “What does it take to break down that nucleus?” Dr. Clark muses. “This model might be able to teach us something about germinal vesicle breakdown and the resumption of meiosis, which is so important for healthy, competent oocytes.”
There is a growing body of science linking rising rates of infertility to environmental factors, from the ubiquity of endocrine-disrupting chemicals to temperature fluctuations associated with climate change. Grasping the basic underlying processes underlying ovarian function through the reconstituted ovary model will allow Dr. Clark and her team to unpack this connection between environmental change and impaired reproduction. “The temperature is going up, we live in an increasingly plasticized world — how does that affect our reproductive tissues and the germline cells?” she asks.
INDEED, RAPID HUMAN-MADE ENVIRONMENTAL CHANGES MAY CONTRIBUTE to the rise in polycystic ovary syndrome, or PCOS, in which the body accumulates excess fat around the midsection. While this was once a clever evolutionary adaptation, the current obesogenic environment has turned it maladaptive, causing metabolic and reproductive dysfunction. This is the theory put forth by Daniel A. Dumesic, MD, professor of reproductive endocrinology and infertility, and an insight that garnered him recognition this year as a Distinguished Researcher by the Androgen Excess and PCOS Society.
Despite affecting an estimated 10-to-15% of pre-menopausal women, PCOS is still poorly understood and underdiagnosed. There are also important subtleties within PCOS, which has multiple manifestations. This confusion and lack of familiarity means that women with PCOS spend an average of more than two years undergoing clinical exams and visiting multiple doctors before being diagnosed.
PCOS wasn’t always such a downer. In fact, Dr. Dumesic notes, millennia ago, extra abdominal fat and infrequent ovulation were valuable evolutionary adaptations. “In times of famine, when we were hunter-gatherers, these women were storing fat, and simultaneously providing glucose and free fatty acids for energy as they hunted,” he explains. “And if they ovulated infrequently, they had less chance of becoming pregnant or dying in childbirth,” giving them and their children a higher chance of survival, albeit within a smaller family. “PCOS, at least in the androgen-excess form [involving elevated levels of testosterone] as we now commonly see, is likely an evolutionary metabolic adaptation.” Unfortunately, Dr. Dumesic adds, in industrialized countries, obesity is far more common than famine, and this predisposition to weight gain no longer serves us. “Our genes can’t keep up with it,” he says.
To buttress this explanation, he and coauthors have documented reports in medical literature of PCOS-like disorders stretching back two millennia, including by illustrious ancient physicians such as Hippocrates and the Middle Ages sage Moses Maimonides. They also cite more modern work on PCOS, such as large genome-wide association studies (GWAS), which allow researchers to scan entire sets of genetic data from across populations to identify genetic variations linked to a specific disease. For PCOS, these genome-wide association studies have examined women with and without PCOS to pinpoint genes that are involved with many reproductive and metabolic functions. Notably, the genes that put women at risk for PCOS are expressed in women found all over the world, pointing to “the ancient human origins of PCOS, potentially dating back before the migration of humans out of sub-Saharan Africa 300,000-50,000 years ago or earlier,” as Dr. Dumesic has written.
Depending on the type of PCOS they have, women can have difficulty getting pregnant or can be highly responsive to the medications used in fertility treatment, including in vitro fertilization (IVF). Younger and leaner women with PCOS can still have good reproductive outcomes because their elevated testosterone level stimulates excess follicle growth. But older PCOS women, or those who’ve already experienced weight gain, will have a harder time, since obesity can pose a risk to oocyte quality.
Knowing this, Dr. Dumesic hopes that patients at risk for PCOS, or for transmitting it to their offspring, can take a more proactive approach to a disease that begins to manifest around the time of puberty. For girls born to mothers with PCOS, this could mean a nutrition and exercise regimen that can help prevent the preferential fat storage that would predispose them to health risks or reproductive issues. For women of reproductive age, especially those who are already at an elevated body-mass index, this could mean prioritizing weight loss long before planning to attempt pregnancy.
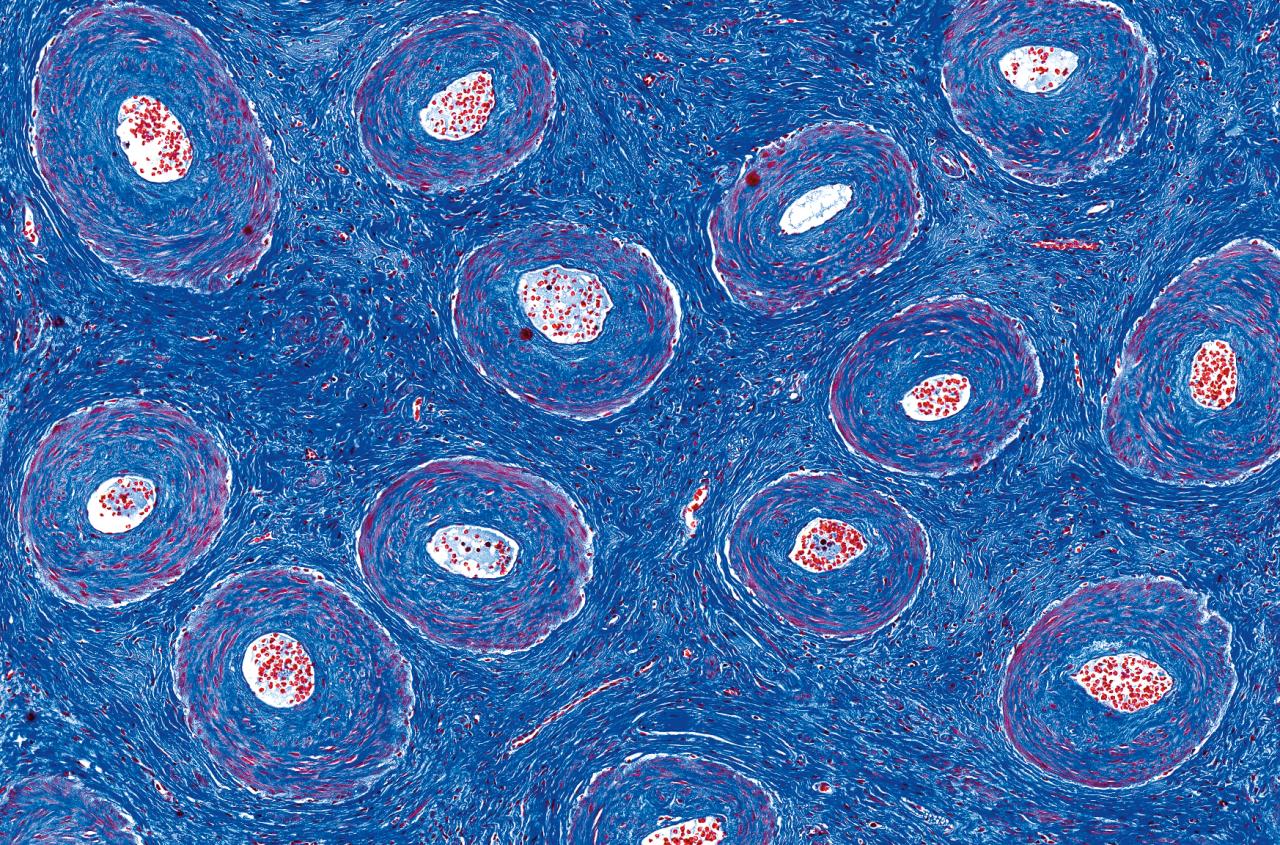
CANCER PREVENTION IS THE HOLY GRAIL FOR CANCER GENETICIST DR. KARLAN, who has been treating patients with ovarian cancer for more than three decades. To date, this goal has proved elusive. “I’ve always viewed ovarian cancer as an albatross around the neck of ob-gyns,” says Dr. Karlan. She was initially inspired to study ovarian cancer as a fourth-year medical student in the 1980s when she encountered a patient perhaps five years older than herself who had an advanced case. As Dr. Karlan recalls, the patient asked her a “very basic question: ‘Why am I here in the hospital dying from ovarian cancer while you are pursuing your dreams to become a doctor?’”
Dr. Karlan took this question to heart, and she has spent her career trying to answer it. At the time, “there was no screening test for ovarian cancer like we have the pap smear for cervical cancer or a mammogram for breast cancer.” Fast forward to today, “and we still don’t have a screening test for ovarian cancer,” she says.
To understand why, it helps to know a few things about this type of cancer, sometimes called “the silent killer.” There are no specific symptoms of ovarian cancer. Its most-common symptoms include abdominal bloating, pelvic pain, feeling full quickly and urinary frequency — symptoms that are so common and vague that they are often ignored or misdiagnosed. In addition, there are many types of ovarian cancer, with distinct characteristics. The most common type, epithelial ovarian cancer, accounts for about 90% of cases, and within that category there are five subtypes. Recognizing that ovarian cancer is not one monolithic disease is helping drive the development of more targeted therapies.
Despite the name, most ovarian cancers are now recognized as originating in the fallopian tubes and not the ovaries. It is believed that very early on the malignant cells from the fallopian tubes migrate to the ovarian surface, where they further grow and metastasize. If physicians were able to catch ovarian cancer at this early stage, survival rates might be closer to 90-to-95%, as they are with early-stage breast cancer. “Part of the problem is a catch-22,” Dr. Karlan says. “Most ovarian cancer presents when it’s already metastatic, at stage 3 or stage 4, and to screen you want to find the disease at stage 1, before it spreads.” (At stage 3, survival rates drop to 35%.) “For us to discover effective screening tests, we need to have access to more early cases.”
“THERE ARE LOTS OF ADVOCATES LOBBYING FOR BREAST CANCER, AND THEY CAN DO IT BECAUSE THEY’RE USUALLY HEALTHY. WOMEN WITH OVARIAN CANCER, THEY ARE TYPICALLY NOT OUTSIDE LOBBYING BECAUSE THEY’RE TOO SICK.”
This creates another catch-22: The lower survival rate may partially explain why less funding has been allocated toward its study than to breast cancer or prostate cancer. (Ovarian cancer is also less common than breast cancer, affecting about one-in-80 women versus breast cancer’s one-in-eight.) “There are lots of advocates lobbying for breast cancer, and they can do it because they’re usually healthy,” says Sandra Orsulic, PhD, a molecular pathologist in the David Geffen School of Medicine at UCLA and longtime collaborator with Dr. Karlan. “Women with ovarian cancer, they are typically not outside lobbying because they’re too sick.”
Dr. Karlan’s work as a clinician informs the research questions she brings into her laboratory work. Just as she could not explain to her patient why she could pursue her dream to become a doctor while the young woman was dying, Dr. Karlan struggled to account for the wide range of clinical outcomes she observed among her patients, even those who had undergone the same surgery on the same day. Why would one be dead within two years while another would be alive and thriving for another 20?
The answers, she thought, lay in the tissues. In 1990, she began a biorepository, a library of human tissues and other samples such as blood, tumor tissue, DNA and urine, annotated with correlative data such as family history of cancer, which treatments they’d undergone and how they’d responded, whether or not they smoked and other potentially relevant information. As time went on, she and colleagues grew the biorepository to include samples of frozen tumors, as well as information about the genetic changes in the tumor and inherited genetic alterations in the patients. It eventually expanded to include more than 100,000 samples. This extensive web of data, alongside daily work with patients, allowed them to test additional hypotheses and identify more connections, and then bring potential insights into the lab to investigate on a molecular basis. “What’s the impact of antibiotics on response to chemotherapy? Did blood pressure medicine or statins impact clinical outcomes? Novel questions that come from clinical observation could be taken back to the laboratory to explore further,” Dr. Karlan says.
When Dr. Karlan began this research in the 1980s, the tools available for laboratory work were far less advanced. But by the early 2000s, geneticists had succeeded in mapping the human genome. As the field of genetics advanced, so did cancer researchers’ understanding of how genes and gene expression contributed to an individual’s susceptibility to cancer, especially with the completion in 2018 of the Cancer Genome Atlas Project, which sequenced samples from more than 11,000 patients with ovarian cancer.
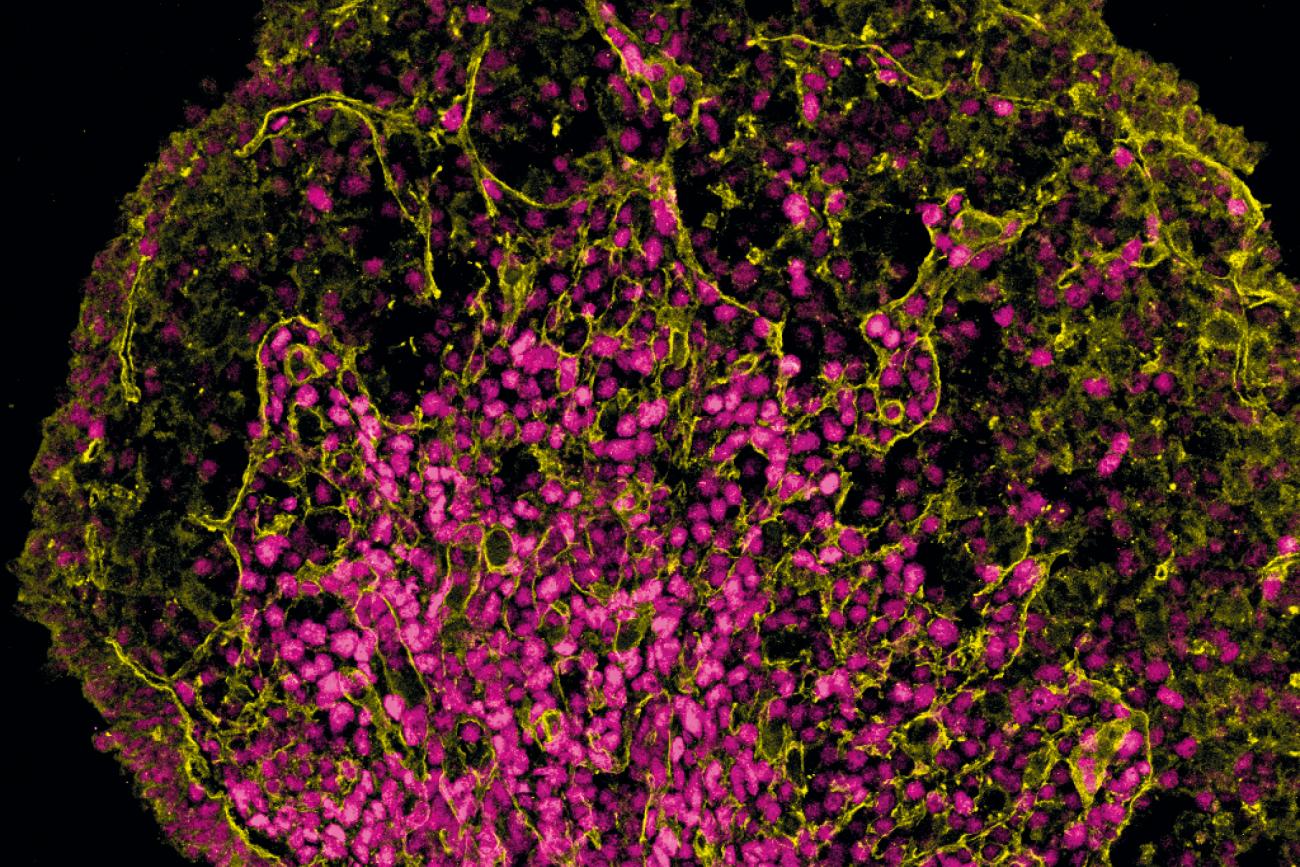
The familial clustering of ovarian cancer that Dr. Karlan and her colleagues had observed was traced to the BRCA1 and BRCA2 genes, which also puts carriers at risk for breast, prostate, pancreatic and other cancers. Interestingly, she and her colleagues found that women with the BRCA mutations tended to have better survival rates from ovarian cancer. “By knowing their genetics, by having their tissues, by knowing the outcomes, we were able to show that women who have these germline mutations in genes that have to do with DNA repair have a better survival rate,” she says.
In BRCA carriers, the mutated gene makes it more difficult for their cells to repair damaged DNA, which leads them to accumulate more defects that can eventually allow cells to transform into cancer cells. However, that same quality makes BRCA carriers more responsive to chemotherapy, which works by causing DNA breaks in the cancer cells themselves. “It’s why they get cancer, but it’s also why it’s easy to kill the cancer cells with therapy,” Dr. Karlan explains.
RESEARCHERS USED THIS SAME INSIGHT TO IDENTIFY A CLASS OF DRUGS CALLED PARP INHIBITORS that are now used to treat ovarian cancer. These drugs block a cell-repairing protein from doing its job, which causes cancer cells with a BRCA mutation to die out. Dr. Karlan noticed that over time, some of her patients were no longer responding to the drugs. Because she had been sampling patients’ tissues longitudinally — for as long as a decade in some cases — she could use genetic sequencing to show how the patients’ cells had mutated over time. This led her and her collaborators to identify “reversion mutations” — changes that occur when the cells become so stressed by chemotherapy and PARP inhibitors that they actually accumulate new mutations that functionally repair the original BRCA mutation, rendering them less sensitive to treatment.
To gain an even deeper understanding of how these changes unfold, Dr. Karlan and Dr. Orsulic turned to mouse models of ovarian cancer, which allow researchers to perform controlled experiments that could not ethically be carried out in humans. When Dr. Orsulic was beginning her career, she also saw that ovarian cancer was under-studied, especially through the use of animal models. Creating one was not a straightforward task. She first attempted to make one as a post-doctoral fellow using mice, long before the Cancer Genome Atlas Project had helped identify relevant genes for ovarian cancer. “We didn’t know what genes to start with,” she says. “It was all pretty anecdotal.”
Furthermore, the mechanics of ensuring that the cancer genes affected only the mouse’s ovaries were incredibly difficult, due to the inaccessibility of the fallopian tube and the sheer tininess of a mouse ovary. Eventually, she removed the mouse’s ovaries, transformed them in a tissue-culture dish with different combinations of mutated genes and put them back into mice to identify which combinations of genetic alterations will lead to cancer development.
Today, she and her colleagues study ovarian cancer tumors on more refined versions of these mouse models, which have been engineered with different mutated genes to exhibit many of the same genetic alterations found in human ovarian cancer. Mice recapitulate many of the aspects of human pathophysiology, including the formation of the tumor microenvironment in reaction to the cancer cells. For example, by examining the microenvironment of tumors, they discovered that following a tumor’s removal, the subsequent wound healing actually attracts remaining free-floating cancer cells to the inflamed tissue where the tumor was cut away. This raises a question: When exactly is the tissue most vulnerable to the re-implantation of these cancer cells? Drs. Karlan and Orsulic have identified the period one-to-five days after surgery as a critical period for intervening to inhibit inflammation, a finding that could help reduce the recurrence of cancer after surgery.
The sheer complexity of the ovary — its role in producing mature, healthy oocytes and preparing the body for reproduction; its vulnerability to dysfunction, whether that be cancer, premature menopause or PCOS; its evolutionary role — means it “needs to be looked at by many different investigators,” Dr. Karlan says. She points to a number of still-unsolved mysteries whose answers may lie within the ovary. Why do some women with a BRCA mutation go through premature menopause? Why do some BRCA carriers get breast cancer at early ages while others get ovarian cancer at a late age? Why does endometriosis increase the risk of ovarian cancer? What’s the role of inflammation?
“There is very, very complex biology at play,” she says. “The ovaries are an extraordinary organ.” With all their mystery and wonder, “the world is a better place because of ovaries.”
