Thomas G. Graeber, Ph.D.
- Professor, Molecular and Medical Pharmacology
- Director, UCLA Metabolomics Center

Thomas G. Graeber, Ph.D., takes an interdisciplinary “systems biology” approach that merges biology, mathematics and computation to identify the Achilles' heels of tumor subtypes. His goal is to identify new ways to diagnose and treat cancer on a patient-specific level.
Graeber seeks to determine how cancer cells evade otherwise effective targeted therapies and to identify new vulnerabilities in the process by which cancers evolve to more aggressive variants. In some cases of cancer, when the main gene driving the cancer is turned off with treatment, the cancer cells can transform into a different cell identity that doesn't rely on that gene.
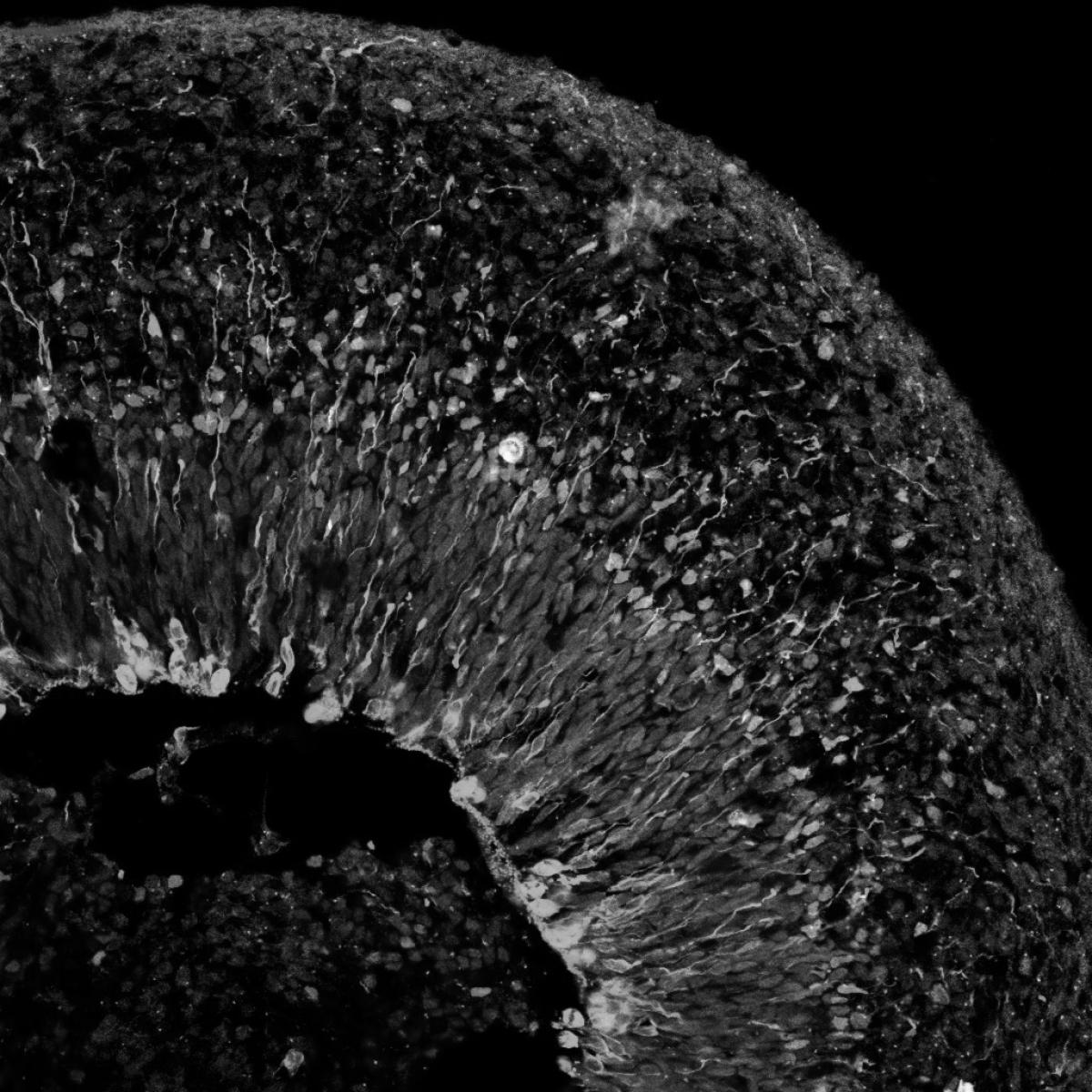
To better understand these changes in cell identity, Graeber studies how cancer cells dedifferentiate, or revert back to an earlier stage of development. Dedifferentiated cells can evade common treatments such as chemotherapy and radiation and cause recurrence of the disease. This research led Graeber to discover that less mature or dedifferentiated melanoma cells showed sensitivity to a self-inflicted cell death called ferroptosis. Certain subtypes of melanoma, Graeber found, could be targeted by a combination of existing cancer therapies and pro-ferroptotic drugs. Parallel aspects are observed in prostate and lung cancers that escape targeted therapy via trans-differentiation. Graeber is pursuing therapeutic approaches to prevent these resistance-enabling changes in cellular identity.
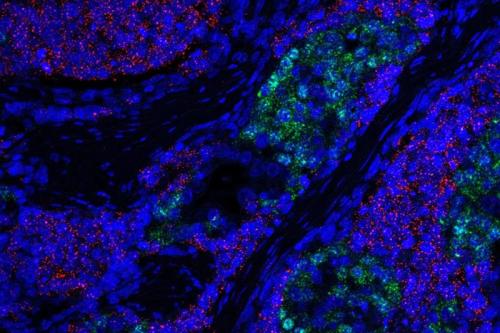
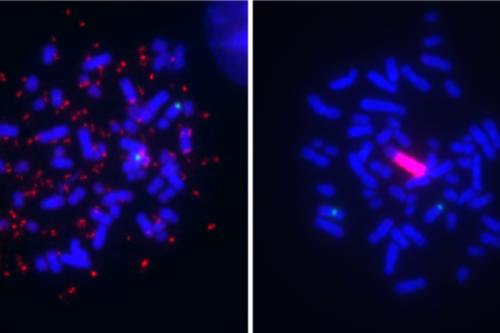
Another novel therapeutic avenue Graeber investigates is how to turn genetic and chromosomal flaws found across aggressive cancer types into a vulnerability rather than a strength. The genomes of aggressive cancers are rife with abnormalities. Cancer cells can use this genomic instability as an advantage to fuel their uncontrolled growth. Graeber is working to identify the mechanisms that enable cancer cells to function despite their flawed genomes to develop drugs to make cancer’s genetic instability a targetable weakness.
Research Projects
- Identifying and inhibiting mechanisms of targeted therapy resistance that occur when cancer cells change their cellular identity through dedifferentiation or transdifferentiation
- Determining how to turn genetic and chromosomal flaws, such as extrachromosomal DNA Short for deoxyribonucleic acid, DNA is a double-stranded molecule that serves as the genetic blueprint for living organisms. Composed of four chemical bases, DNA encodes the instructions necessary for protein synthesis and governs the development, function, and inheritance of traits in an organism. DNA Short for deoxyribonucleic acid, DNA is a double-stranded molecule that serves as the genetic blueprint for living organisms. Composed of four chemical bases, DNA encodes the instructions necessary for protein synthesis and governs the development, function, and inheritance of traits in an organism., found across aggressive cancer types into vulnerabilities rather than strengths
- Determining how cancer cells alter their metabolism to drive aggressive phenotypes
- Defining the mechanisms that drive highly undifferentiated sarcomas, including genomic instability and stem cell programs
-
Post-doctoral Fellowships
- Computational Biology, UCLA, 2004
- Signal Transduction, UCLA, 1999
Degree
- Ph.D., Physics/Cancer Biology, Stanford University, 1996