
Clinical trial led by UCLA receives $7.4 million in state stem cell funding
This morning, the California Institute for Regenerative Medicine voted unanimously to award UCLA with $7.4 million to lead a phase I/II clinical trial for X-linked chronic granulomatous disease, an immunodeficiency disorder commonly referred to as X-linked CGD. The trial, which will use stem cell gene therapy to correct the genetic defect associated with the disorder, is led by renowned stem cell researcher and UCLA Eli & Edythe Broad Center of Regenerative Medicine and Stem Cell Research member, Dr. Donald Kohn.
“CIRM has provided us with a very exciting opportunity to assess the viability of a potential cure for this devastating disease using a novel therapy that, if successful, could possibly be utilized in other diseases in the future,” said Dr. Kohn, professor of pediatrics in the UCLA David Geffen School of Medicine, professor of microbiology, immunology and molecular genetics in Life Sciences at UCLA, member of the UCLA Children’s Discovery and Innovation Institute at Mattel Children’s Hospital and the principal investigator on the trial.
During the meeting, CIRM Chairman Dr. Jonathan Thomas highlighted that the grant reviewers expressed “considerable enthusiasm for the science and [Dr. Kohn’s] team.” Members of the CIRM grants review committee added that Dr. Kohn put together a clinical trial of “exceptional merit.”
The trial will enroll 10 patients who will receive the stem cell gene therapy at one of three U.S. sites: Mattel Children’s Hospital UCLA, Dana-Farber/Boston Children’s Cancer and Blood Disorders Center and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health at the NIH Clinical Center in Bethesda, Maryland. As the sponsor of the clinical trial, UCLA will oversee all U.S. research sites.
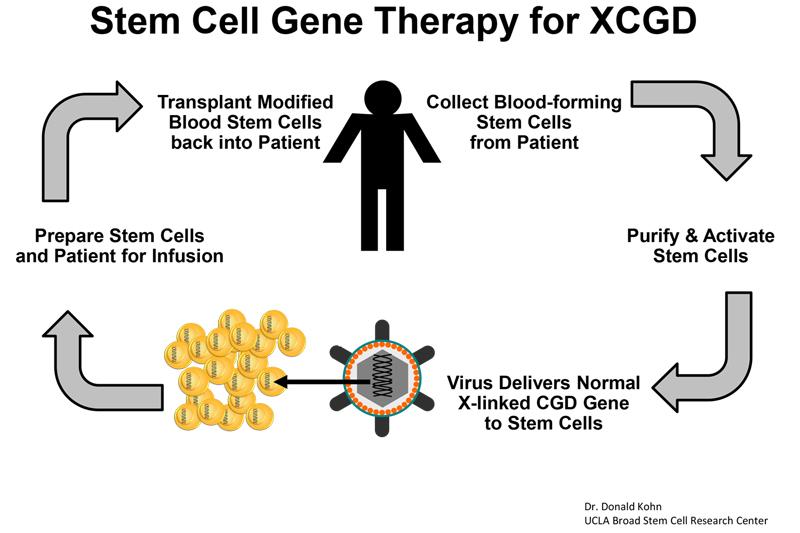
The trial is being run in partnership with Genethon, a nonprofit biotherapy research and development organization in France that engineered the virus delivery system designed to transport the gene therapy into the patients' blood-forming stem cells. Genethon is sponsoring a parallel European clinical trial that is currently open and recruiting in three cities (London, Frankfurt and Zurich) and is also preparing to open in Paris. The European trial sites utilize the same gene therapy to be used in Dr. Kohn’s trial.
Chronic granulomatous disease, which prevents white blood cells from effectively killing foreign invaders such as bacteria, fungi and other microorganisms, is an inherited blood disorder affecting approximately 1 in 200,000 persons in the U.S. At least five different gene mutations are linked to CGD. The disorder is usually diagnosed before age 5 and can cause severe, persistent and untreatable tissue infections that lead to the formation of granulomas (a mass of tissue produced in response to infection). However, people with CGD may experience a range of severity in symptoms. Without treatment, children often die within the first decade of life. The most common type of CGD occurs in a gene on the X-chromosome, meaning it only occurs in males; 65 percent of CGD patients have X-linked CGD.
“We will start by treating the most common form of the disorder, X-linked CGD,” said Dr. Caroline Kuo, clinical instructor in the Division of Allergy & Immunology, Department of Pediatrics at UCLA and chair of the study. “Our hope is that this treatment will provide a lasting cure for X-linked CGD patients, whose quality of life is greatly impacted by the disorder. If it works, the treatment could theoretically be expanded to treat all forms of CGD.”
The stem cell gene therapy process used in the trial will use patient’s own blood-forming stem cells. Blood-forming stem cells are capable of turning into any type of blood cell, including the white blood cells central to the immune system. Blood stem cells will be removed from the patients and modified to correct the genetic defect that causes X-linked CGD. A virus delivery system created by Genethon will be used to insert the corrected gene. Once treated, the modified blood stem cells will be transplanted back into the patient, with no risk of rejection since the gene therapy uses the patient’s own cells. If the treatment is successful, the blood-forming stem cells will produce white blood cells without the X-linked CGD genetic mutation, meaning the patient’s immune system will have the capability to fight infection.
The phase I/II X-linked CGD clinical trial is designed to assess treatment safety. Over the course of two to three years, Mattel Children’s Hospital UCLA and Dana-Farber/Boston Children’s (led by Dr. David A. Williams) will each enroll three patients; the NIH Clinical Center (led by Dr. Elizabeth Kang) will enroll four patients. Further trial details can be found on the NIH’s clinicaltrials.gov website.
The X-linked CGD gene therapy is investigational and has not yet been approved by the U.S. Food and Drug Administration as safe and effective for use in humans; it may be used only in research studies.

