Andrew S. Goldstein, Ph.D.
- Associate Professor, Molecular, Cell and Developmental Biology
- Associate Professor, Urology
- Chair, Biomedical Research Minor

Andrew Goldstein, Ph.D., investigates the role prostate epithelial cells play in development, cancer formation, reproduction and aging. He seeks to improve our understanding of the mechanisms driving prostate cancer initiation, progression and treatment-resistance, which could lay the foundation for new therapies and methods for early detection.
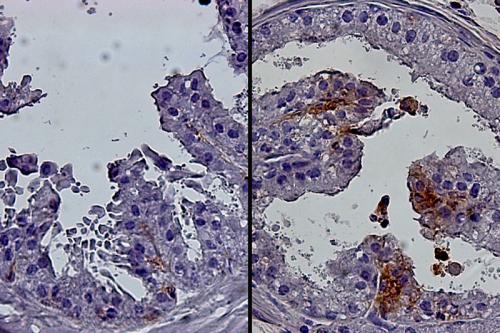
Goldstein’s research is focused on the intersection between cancer biology, aging and metabolism. Early in his research career, he described the isolation of epithelial progenitor cells — which give rise to the cells of the epithelial tissues that cover many surfaces both inside and outside of the body — from mouse and human prostate tissue. This work demonstrated the capacity of progenitor cells to initiate prostate cancer in response to oncogenic transformation.
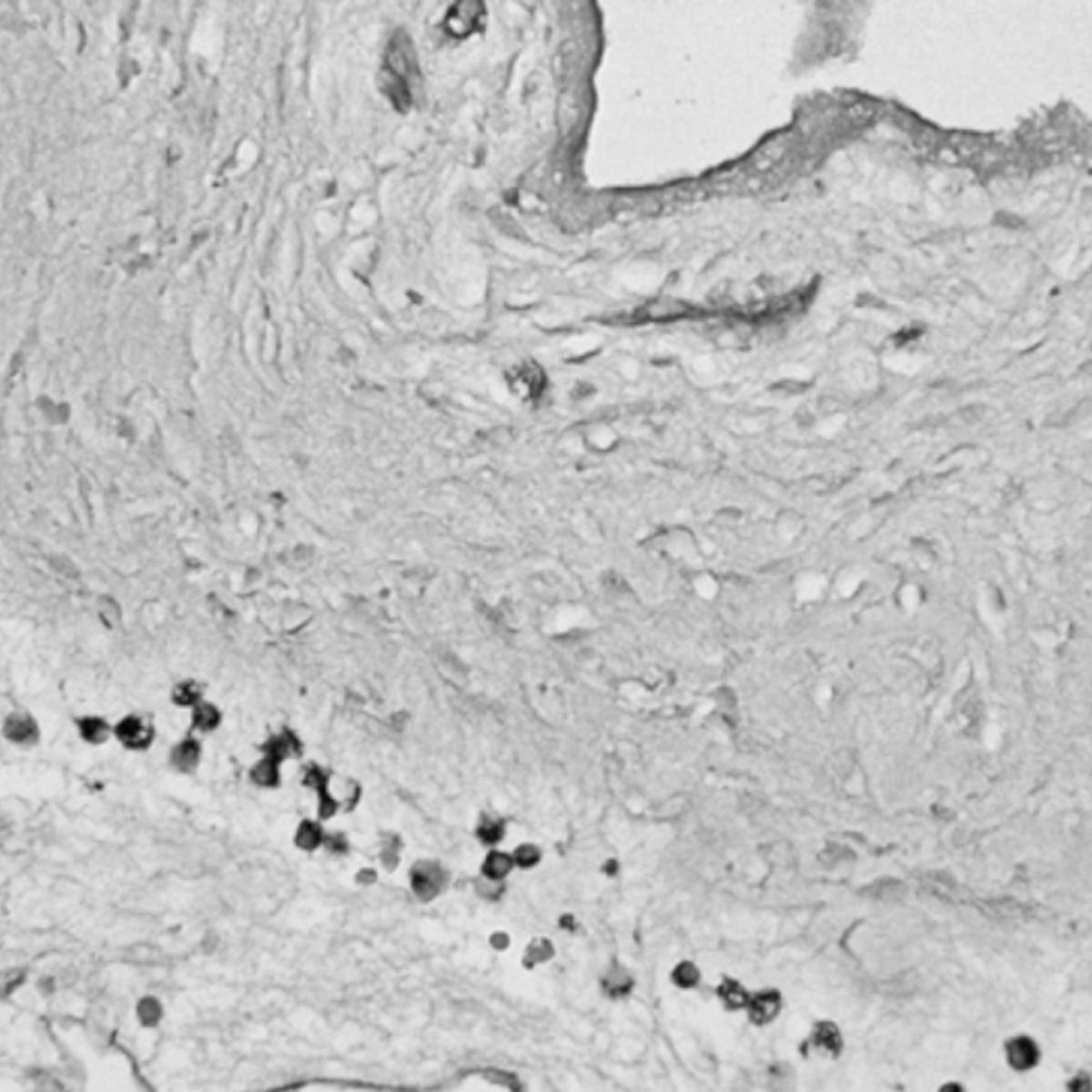
He also demonstrated that chronic inflammation in the human prostate is associated with an expansion of rare progenitor-like luminal cells that can initiate aggressive prostate cancer. These findings help to explain why chronic inflammation increases prostate cancer risk. He also determined that these luminal progenitor cells are increased in the aging mouse and human prostate, providing a potential explanation for the age-related increased risk of disease in this gland.
His recent work has yielded new insights into prostate cancer metabolism, the process by which these cells harness energy to grow and spread. Changes in metabolism, Goldstein found, can influence the “genetic instructions” within prostate cancer cells that determine how they develop and become resistant to hormone therapies. These discoveries could be leveraged to inform the development of new therapeutic approaches that make aggressive tumors more treatable.
Goldstein is deeply committed to mentoring his students and fostering an inclusive and supportive research environment. In addition to training his students in unbiased experimental design, methodology, analysis, interpretation and reporting of results, he assists them in devising strategies to reach their career goals.
Research Projects
- Defining metabolic regulation of luminal differentiation The process by which stem cells transform into specific, specialized cell types with distinct functions and features. differentiation The process by which stem cells transform into specific, specialized cell types with distinct functions and features. in prostate cancer
- Investigating the relationship between prostate cancer metabolism and resistance to hormone therapies
- Determining the role of aging in susceptibility to prostate cancer initiation
- Developing new models of human prostate cancer initiation and progression
-
Degree
- Ph.D., Molecular Biology, UCLA, 2011
-